Marine Lines, Mumbai, Maharashtra
- GST NO. : 27AABPR4112P1ZH
| Business Type | Manufacturer, Exporter, Supplier, Retailer, Trader, Distributor, Importer, Buying House |
| Type | Box |
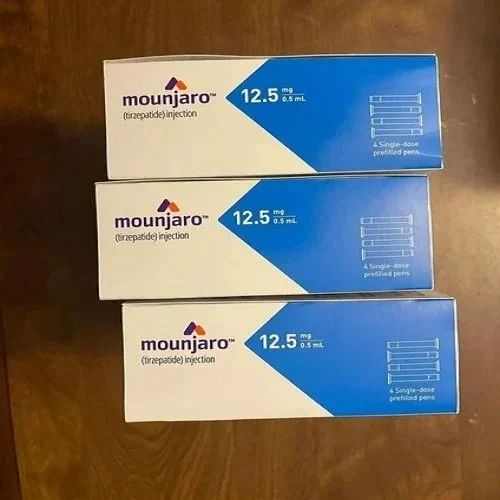
| Brand | Mounjaro Tirzepatide Injection |
| Material | Injection |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
Product Description
Eli Lilly and Company is proud to announce that the U.S. Food and Drug Administration (FDA) approved Mounjaro™ (tirzepatide) injection for the treatment of adults with type 2 diabetes as an adjunct to diet and exercise. The approval of Mounjaro represents the first new class of diabetes medication introduced in nearly a decade.Mounjaro is the first and only approved once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist. It is a single molecule that targets the body's receptors for GIP and GLP-1, which are natural incretin hormones. Results from the global phase 3 SURPASS program supported this approval.
Looking for "Mounjaro Tirzepatide Injection 12.5 Mg Box" ?
Explore More Products